Meet the new

Neurodyn
Evolution
Rehabilitation of
urogynaecological
and coloproctological
disorders

Discover the new Neurodyn Evolution
Treatments for incontinence, overactive bladder, and pelvic floor muscle dysfunctions.
Now you have total control of treatments in the palm of your hand!

Control by App
Developed to adjust therapies, demonstrate patients’ contraction level via biofeedback, and provide greater practicality for users.
*
Currently, the app is available only for computers and tablets, minimum screen size 8.7″ or larger, with specific configurations (minimum resolution of 1024×600 pixels, and Quad-core 1.3 GHz processor or higher). Currently not compatible with iOS devices. We are developing new versions and will bring news soon.
Therapeutic
Modalities
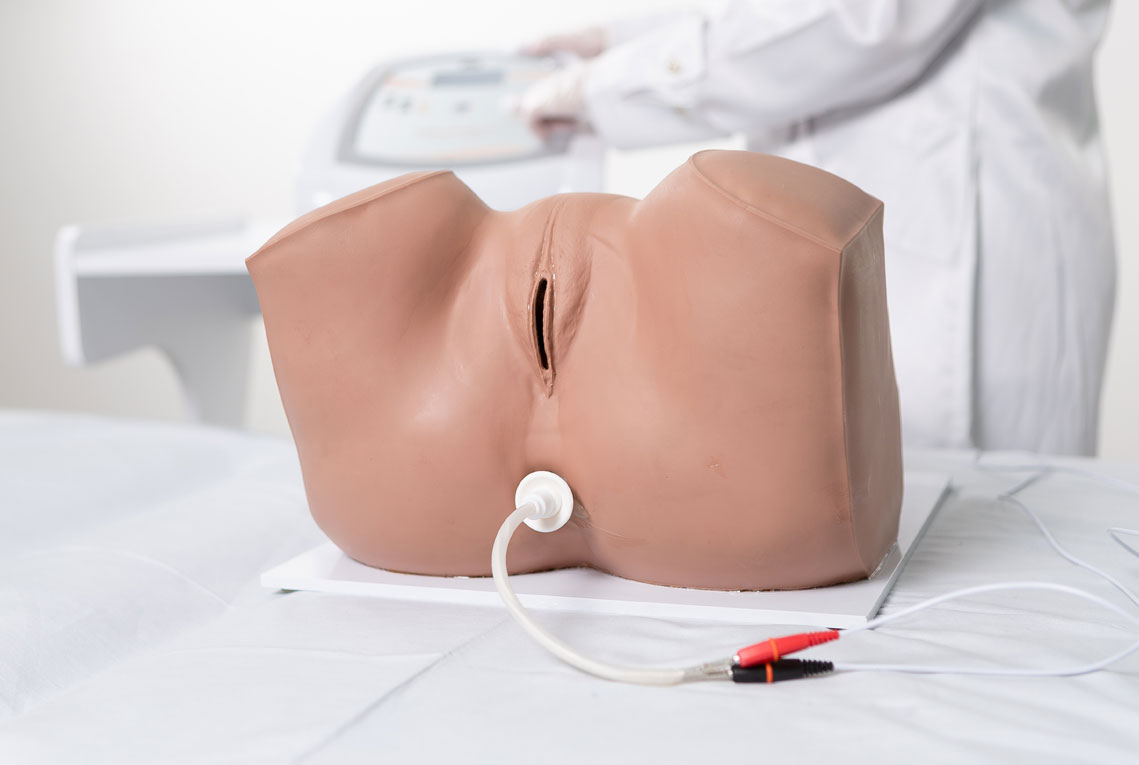
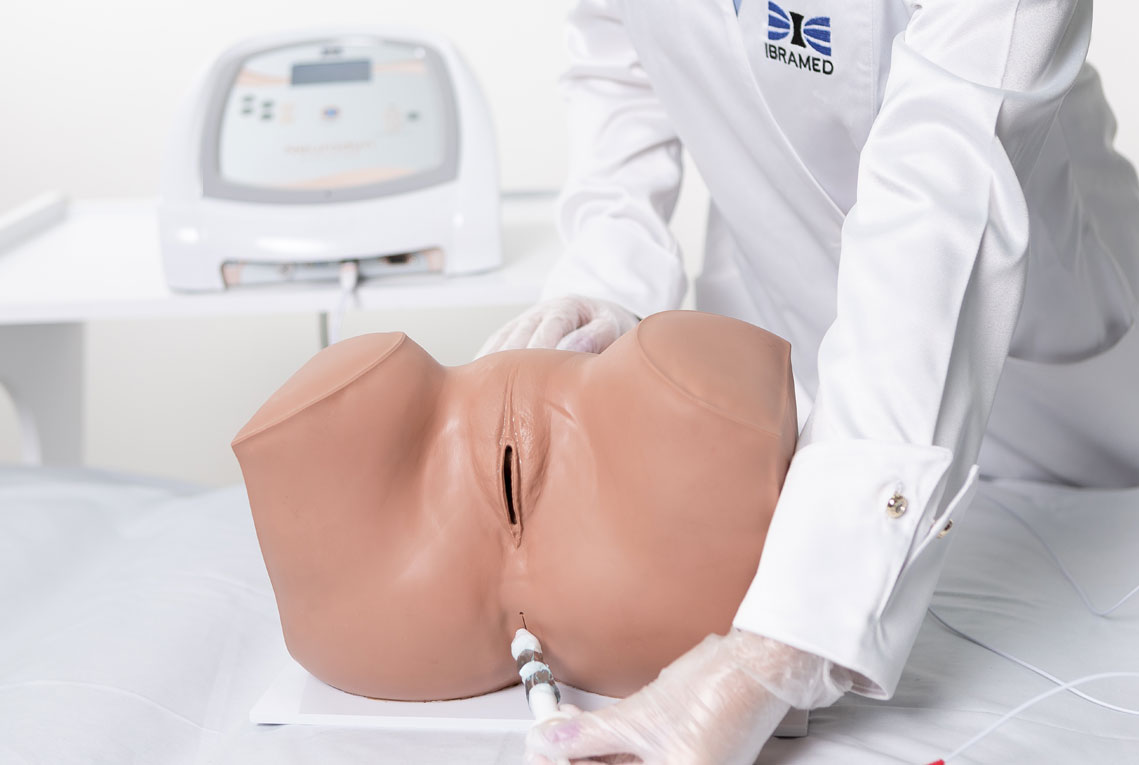
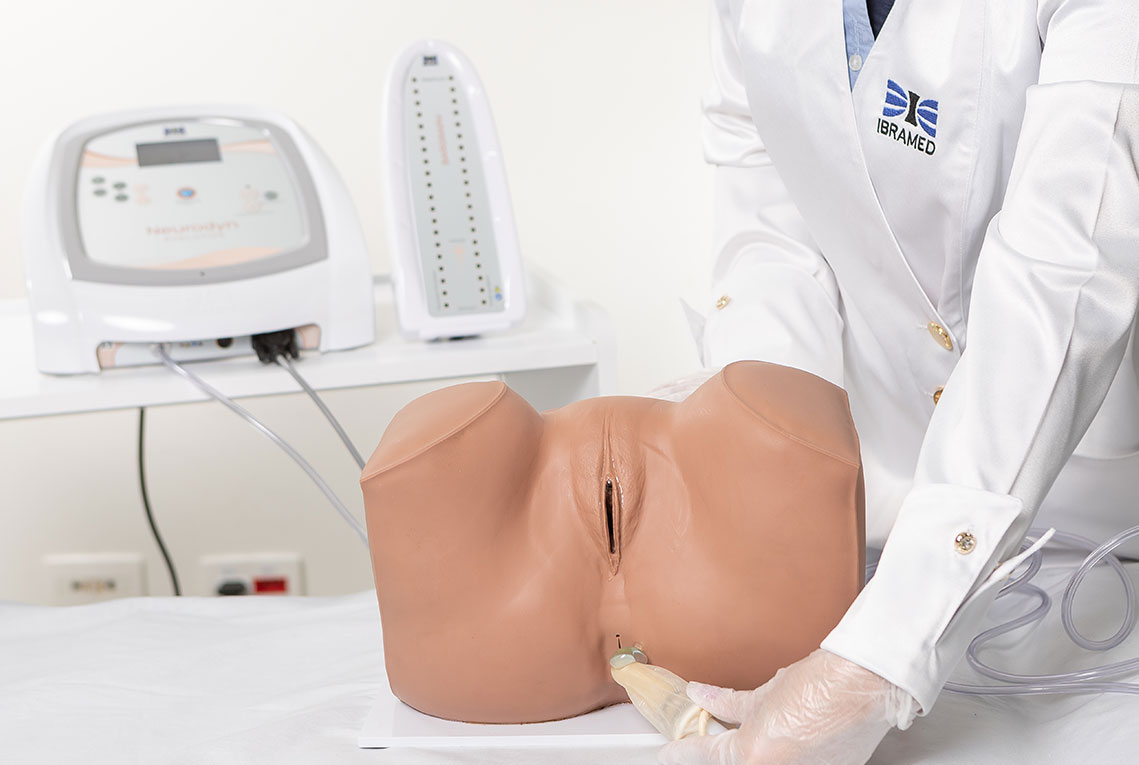
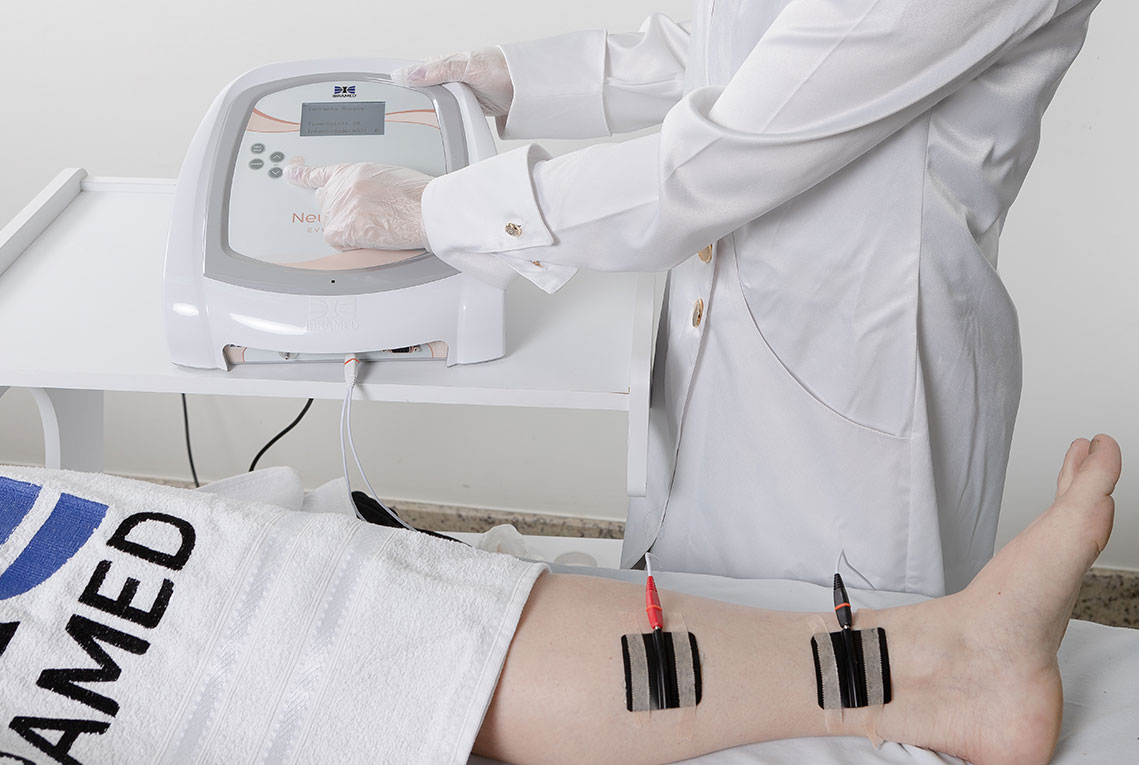
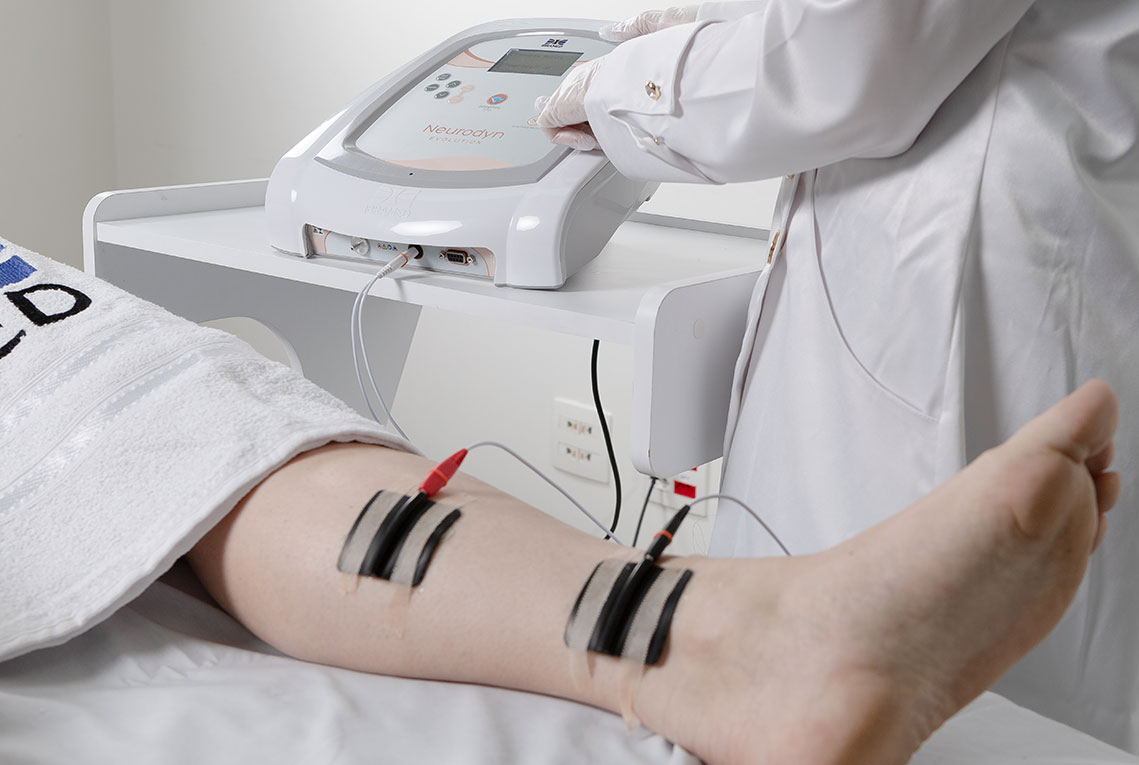
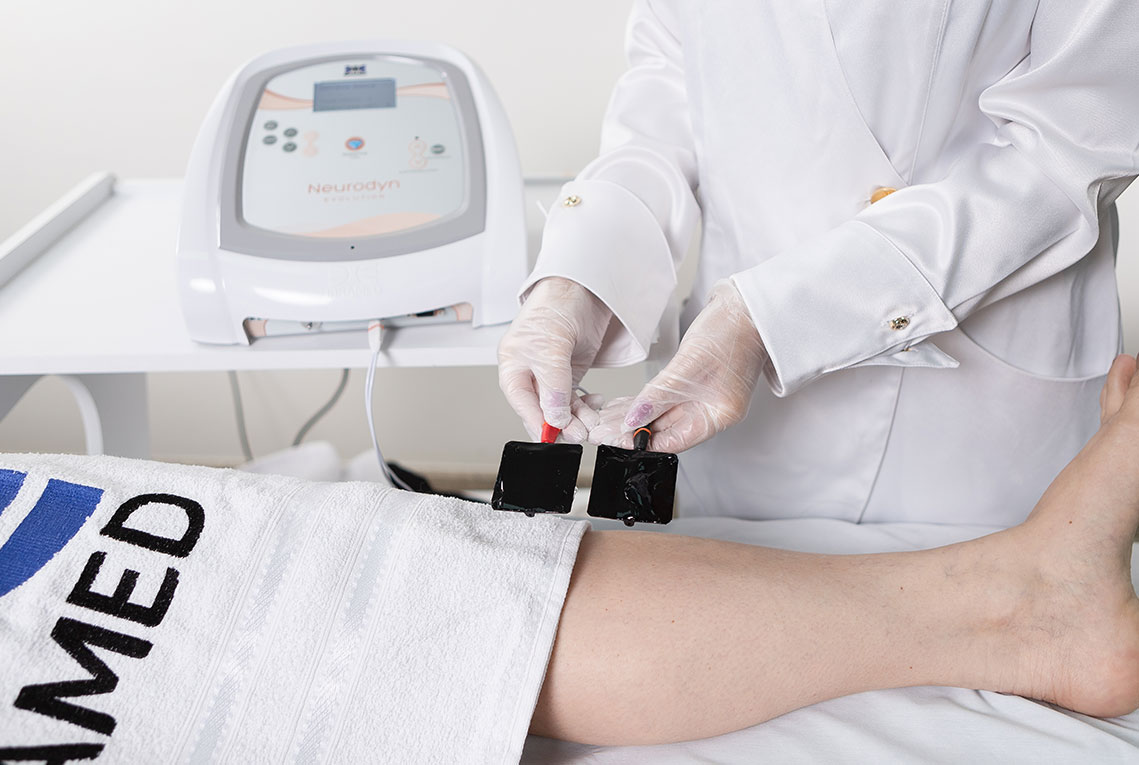
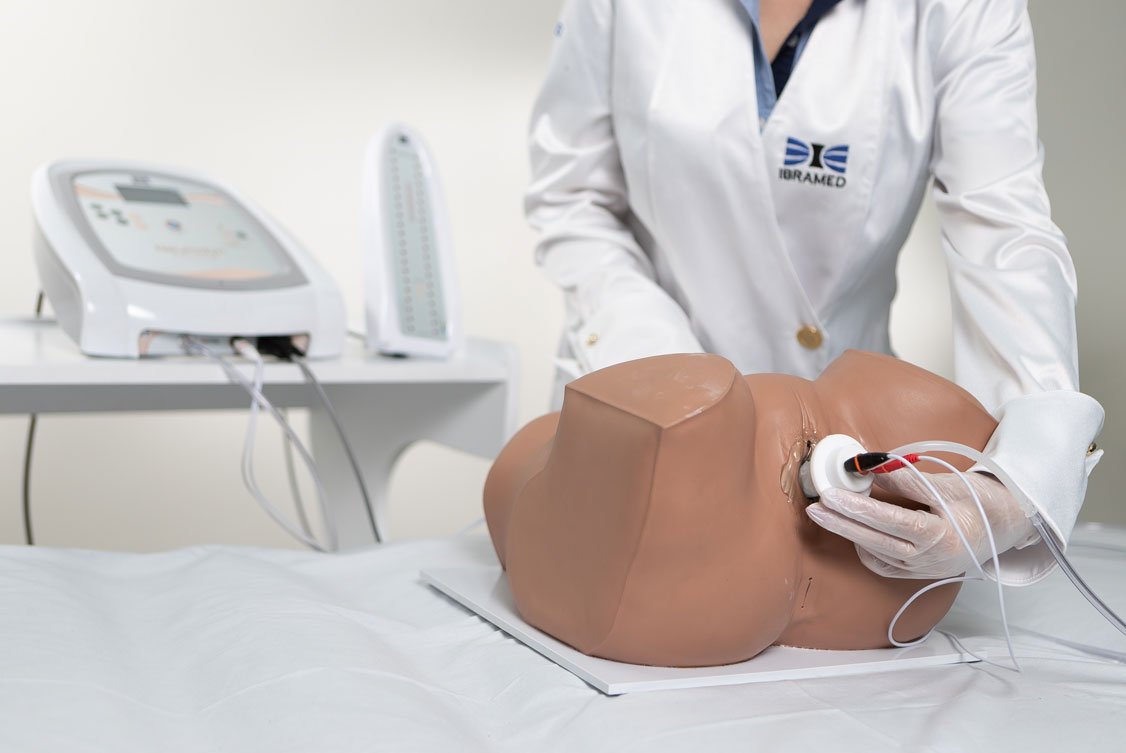
1. Pelvic Floor Electrostimulation
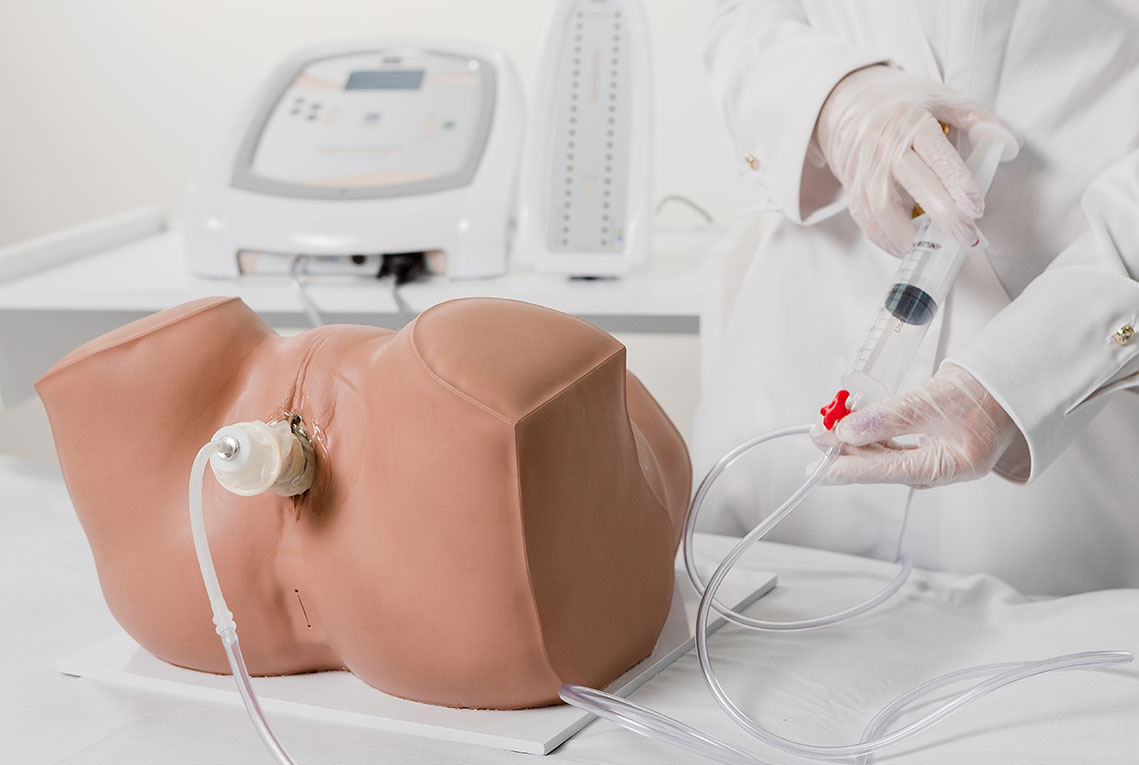
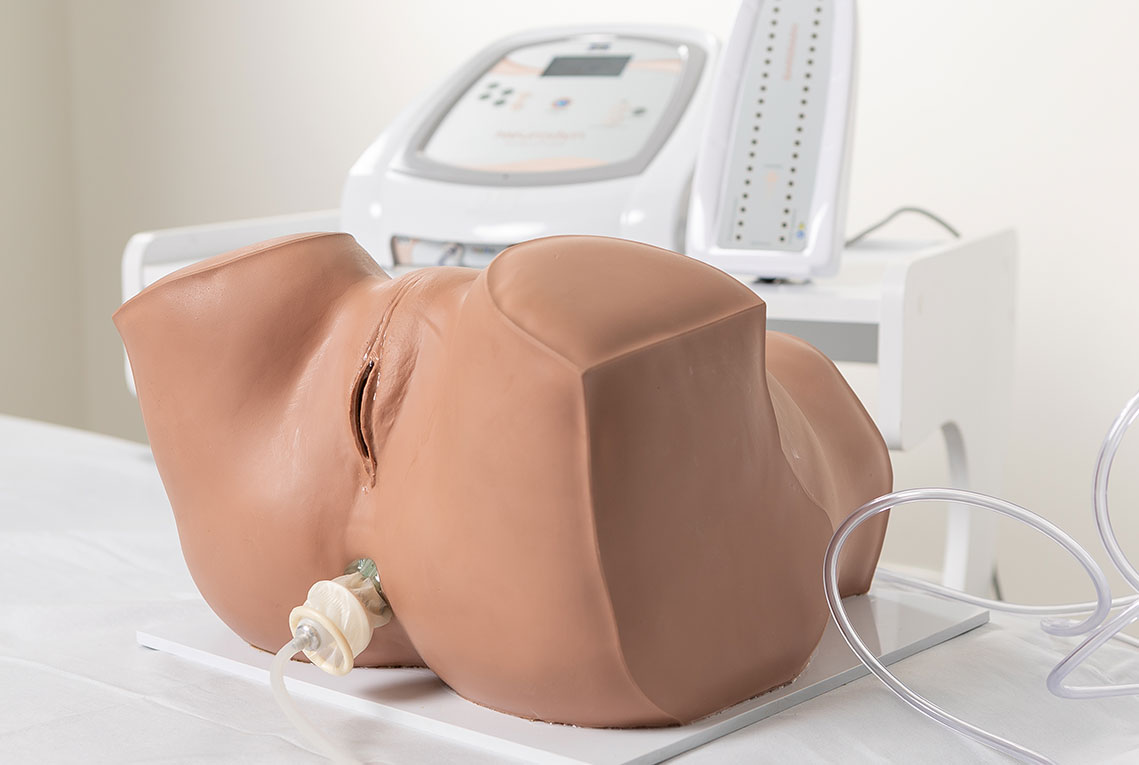
Intravaginal or Intra-Anal Applications
Pelvic floor electrical stimulation is indicated for patients who do not recognize the perineal muscles and does not require active participation. Once the patient can contract the muscles adequately, this therapy can be combined with kinesiotherapy or biofeedback to increase its effectiveness. In addition to improving muscle function, there is also an enhancement of body awareness through learning how to properly contract the pelvic floor muscles.
Electric currents:
(Functional Electrical Stimulation)
(Transcutaneous Electrical Nerve Stimulation)
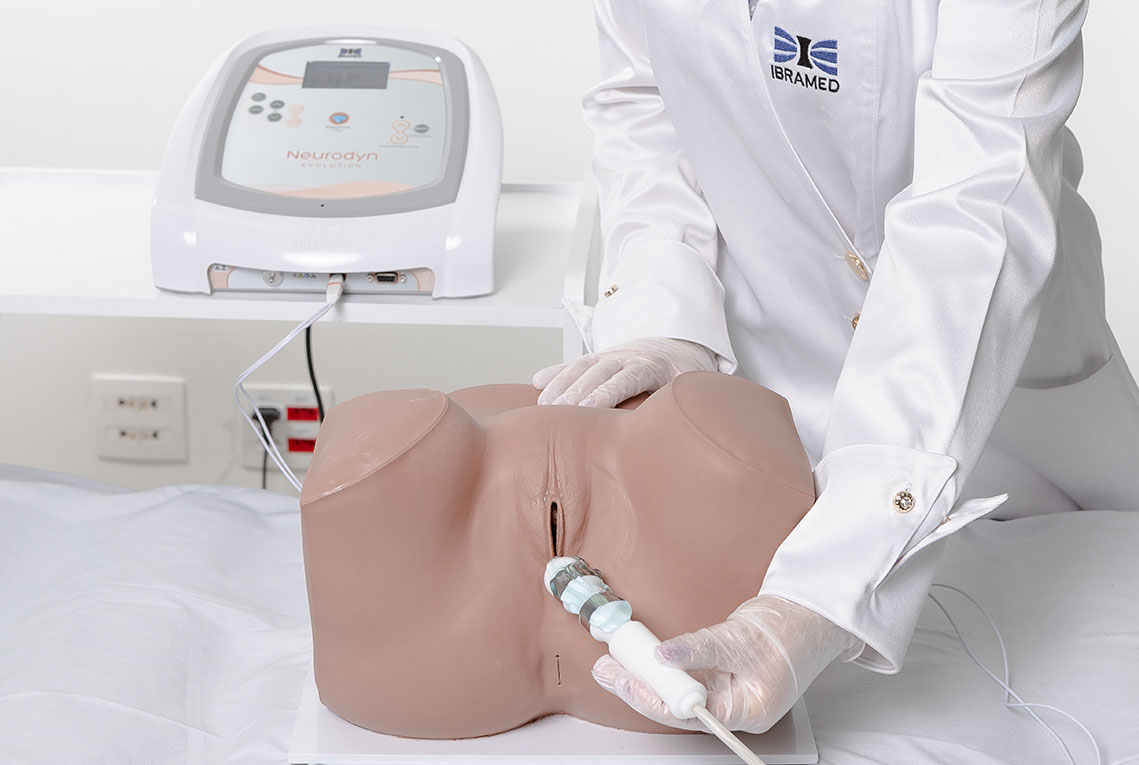
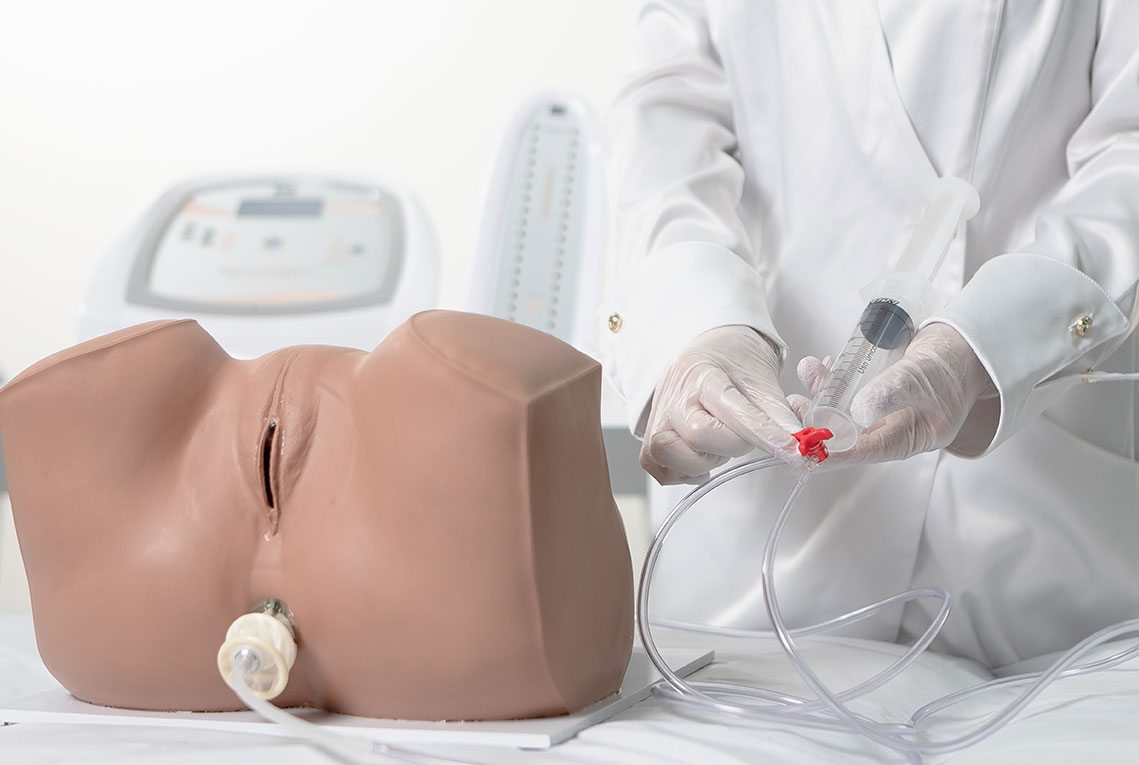
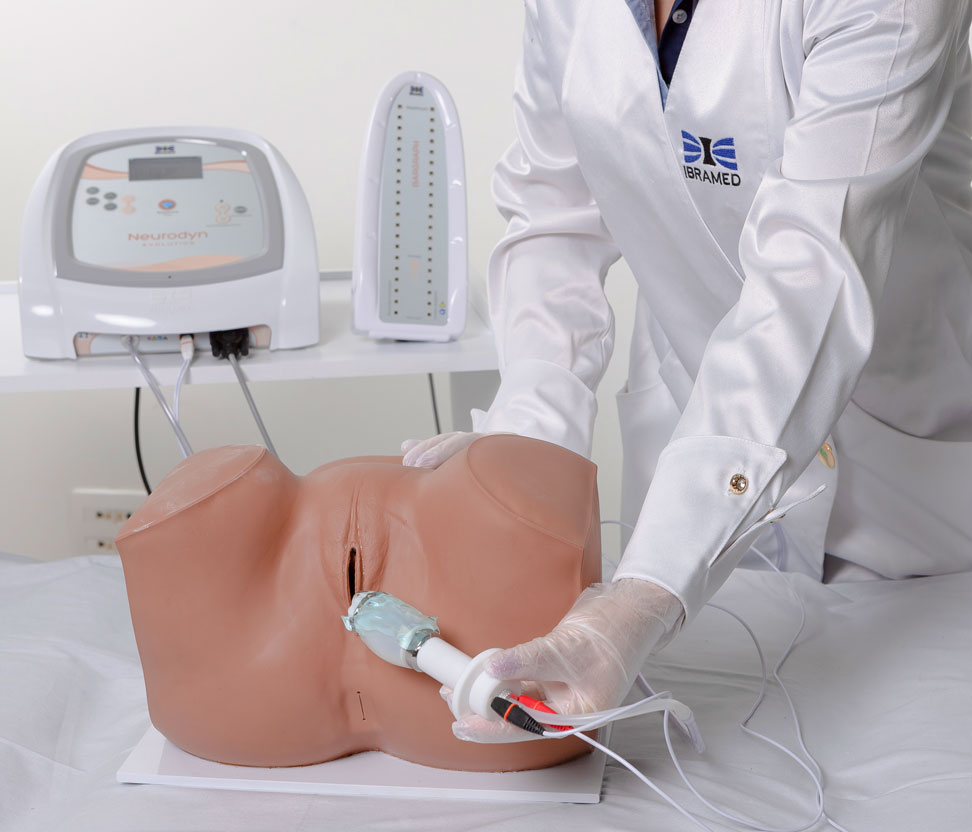
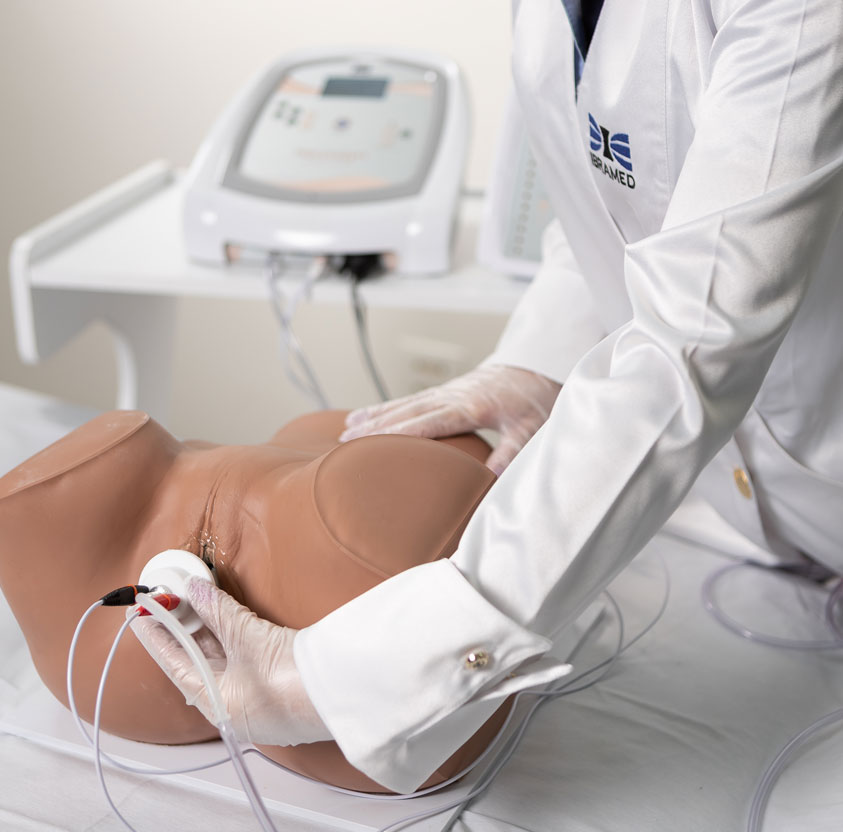
2. Biofeedback
Intravaginal or Intra-Anal Applications
Biofeedback is performed by placing inflatable vaginal or anal probes that inform the patient via visual signals whether the perineal contraction is correct. The goal is for the patient to learn which muscle groups should be contracted, increasing voluntary control. The inflatable probe is connected to a microprocessor that provides immediate feedback regarding the muscular contraction level, reflecting the intensity and strength. Specific exercises help identify and strengthen the pelvic floor muscles, providing visceral support and improving incontinence.
3. Transcutaneous Electrostimulation of the Tibial Nerve
Peripheral Electrostimulation
Combined
Therapies
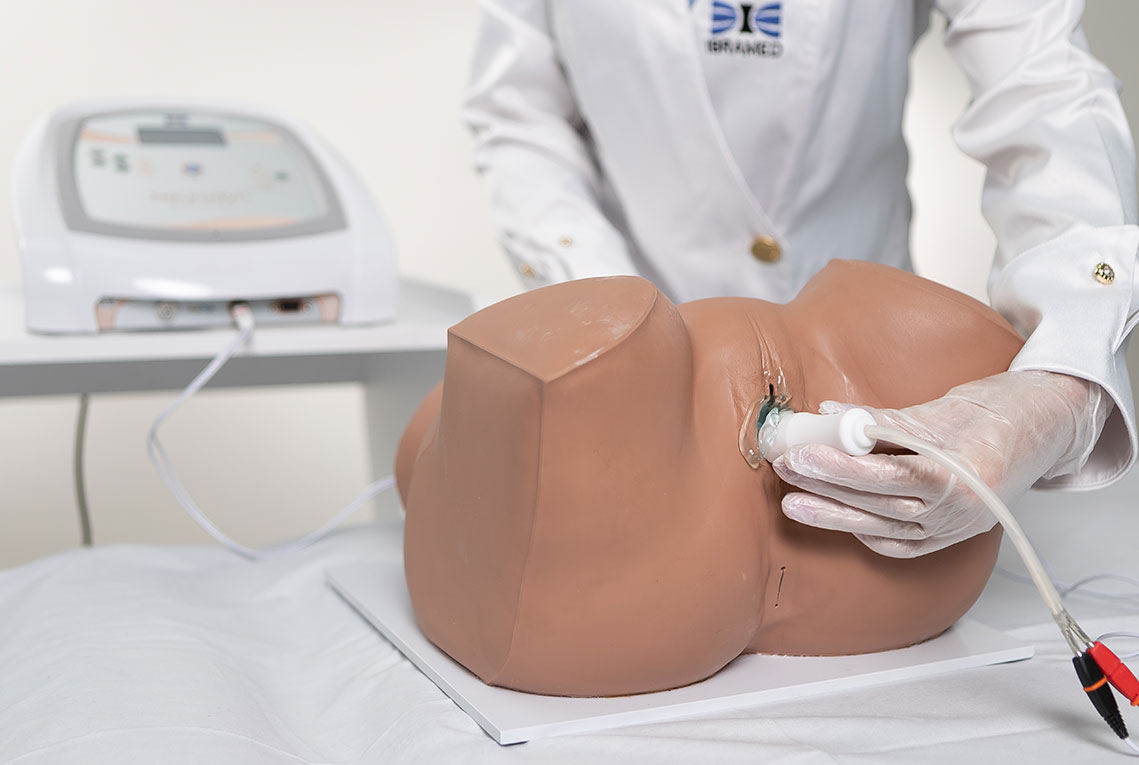
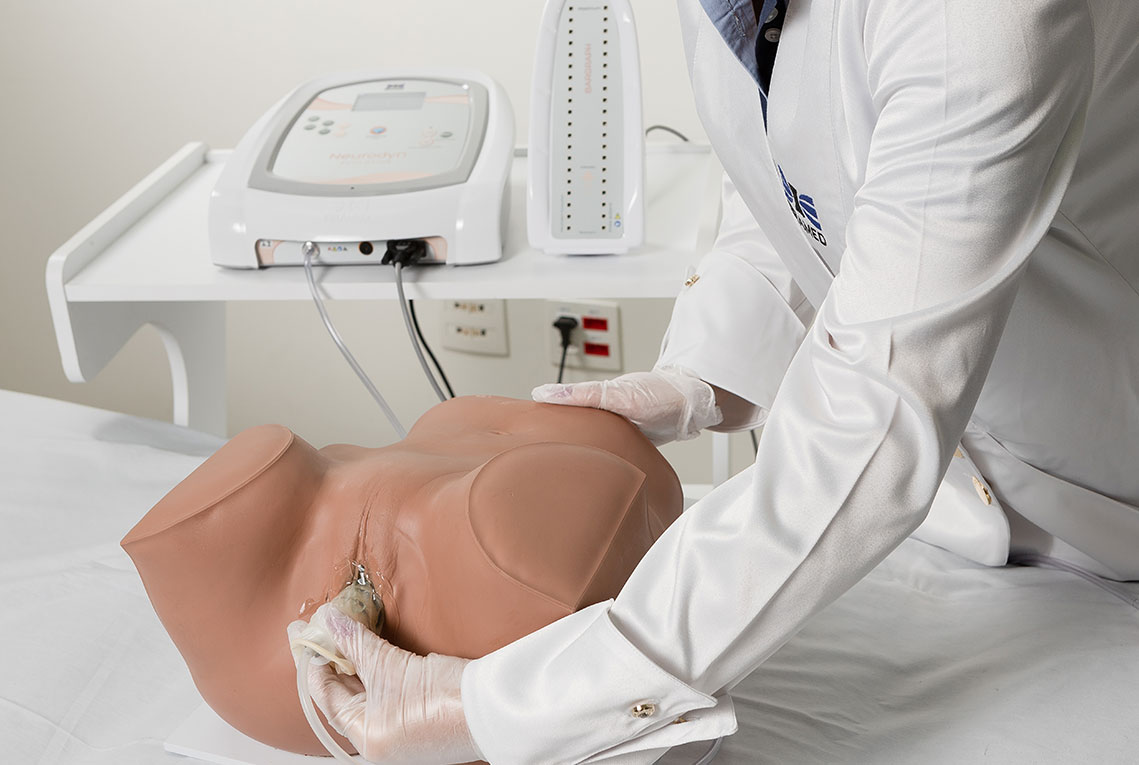
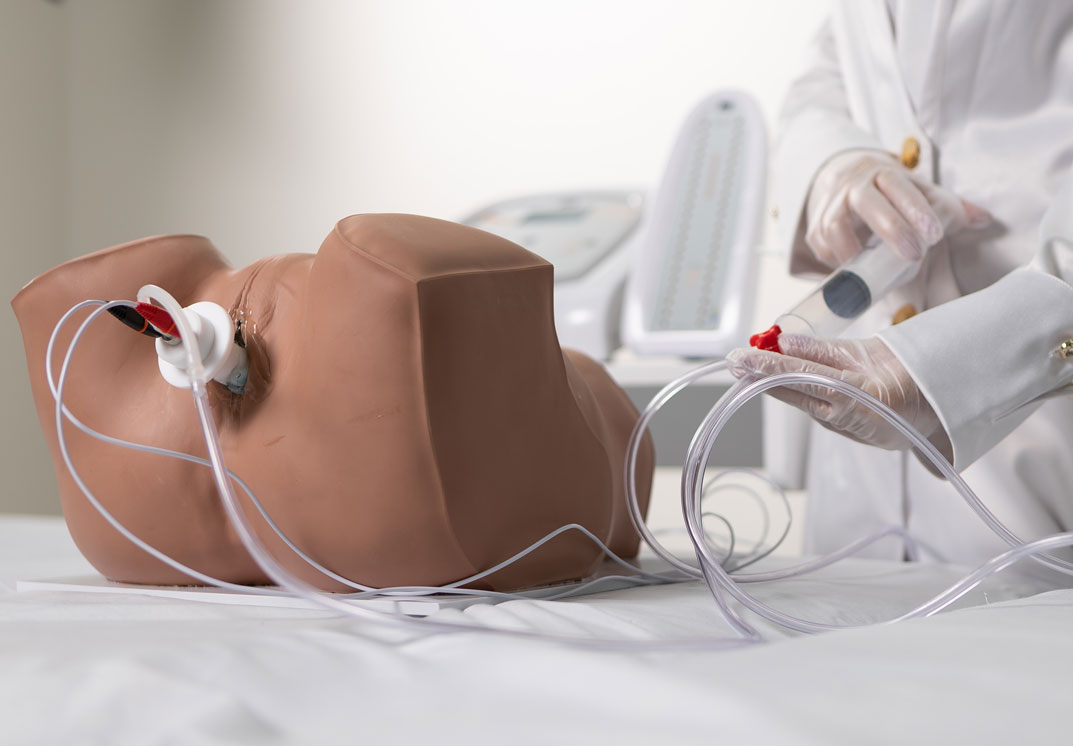
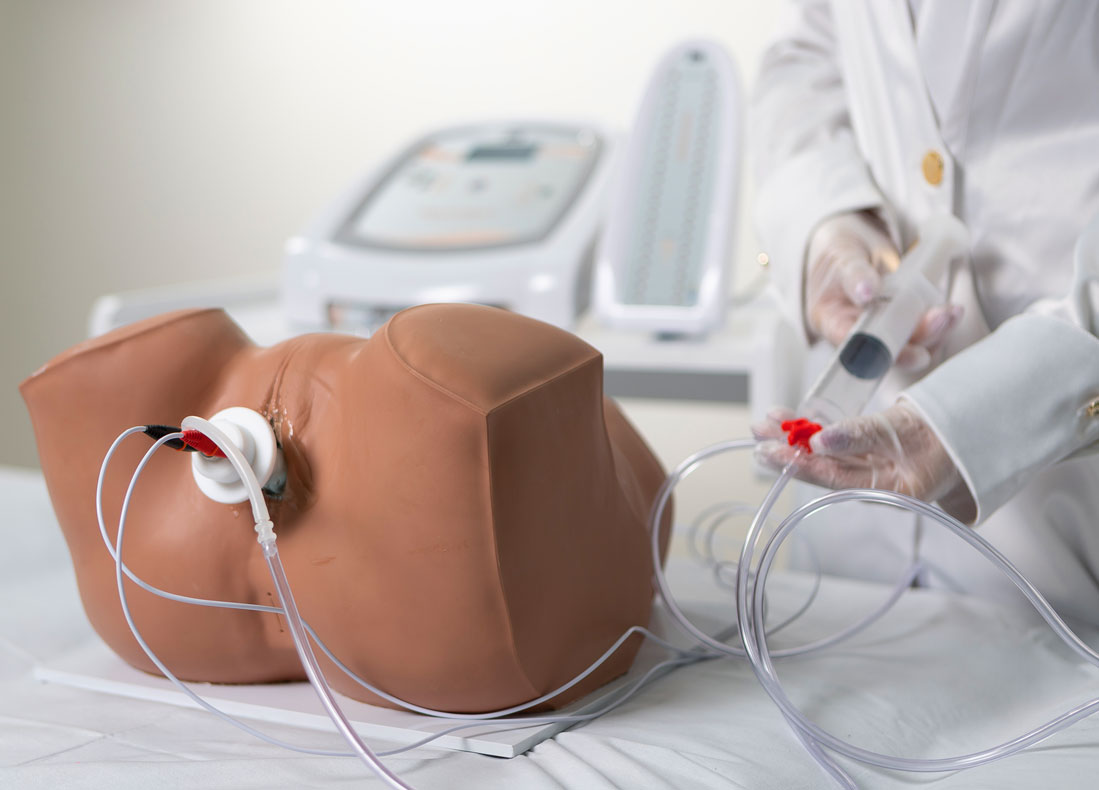
4. Biofeedback + Electrostimulation (Aussie or FES)
Based on the therapeutic effects of both electrostimulation and biofeedback, IBRAMED developed an exclusive probe for a new application. The objective is to combine both techniques simultaneously at the same site: when the electrostimulation stimulus occurs, the device measures perineal contraction strength to enhance clinical results and optimize treatment.
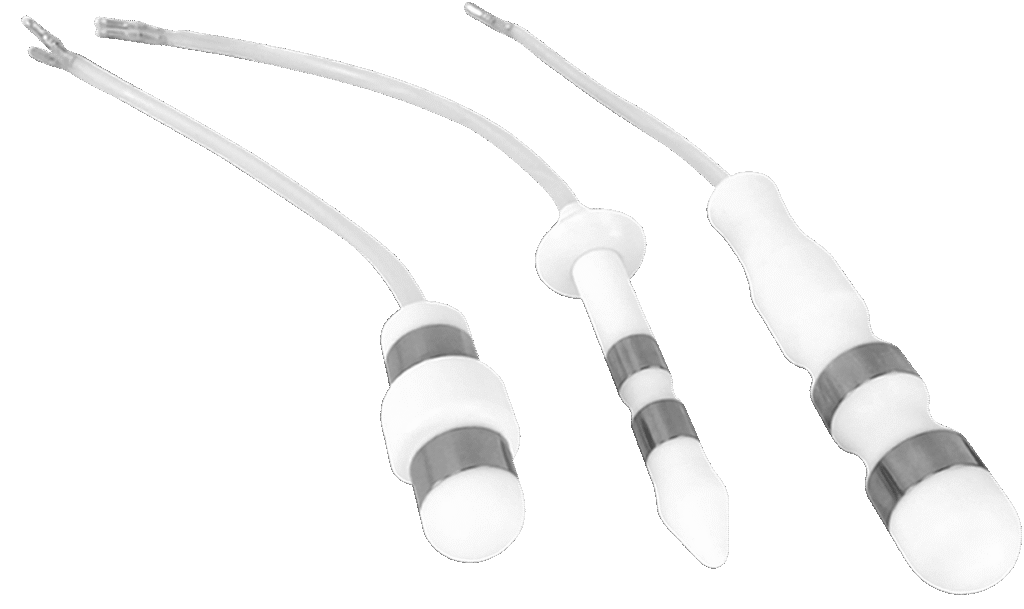
Probe(s) used:
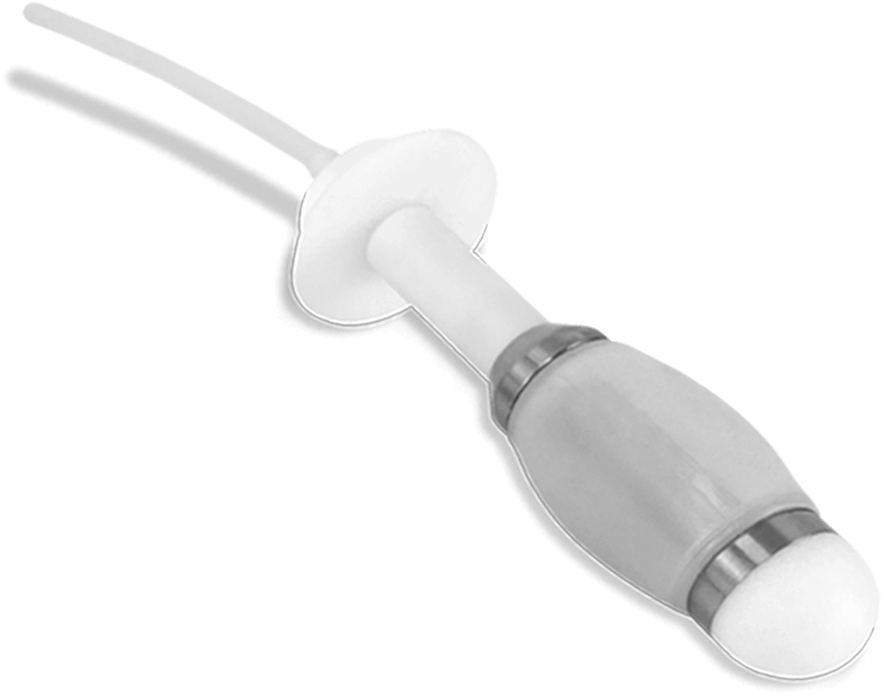
Probes
that come with
the equipment
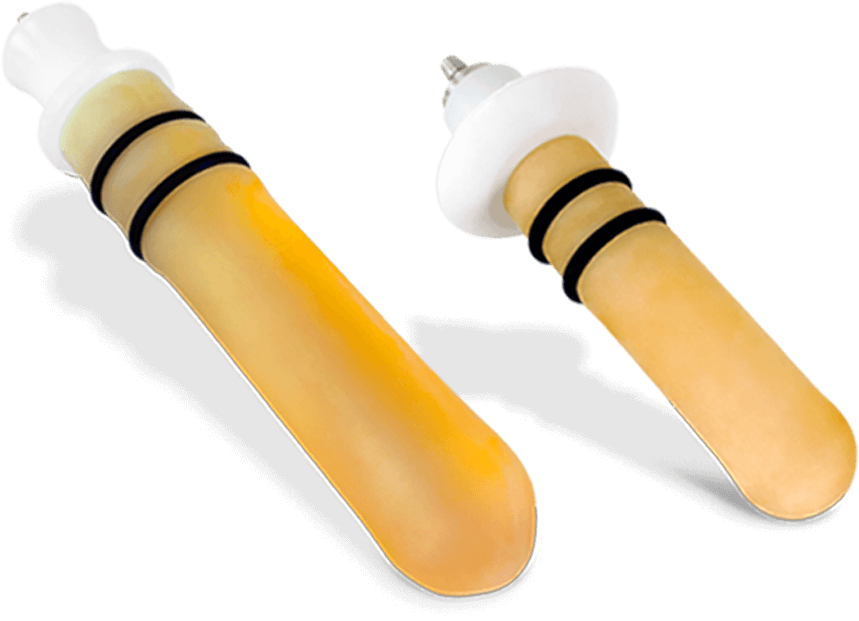
Teflon probes

Teflon + Latex combined therapy probe

Latex probes


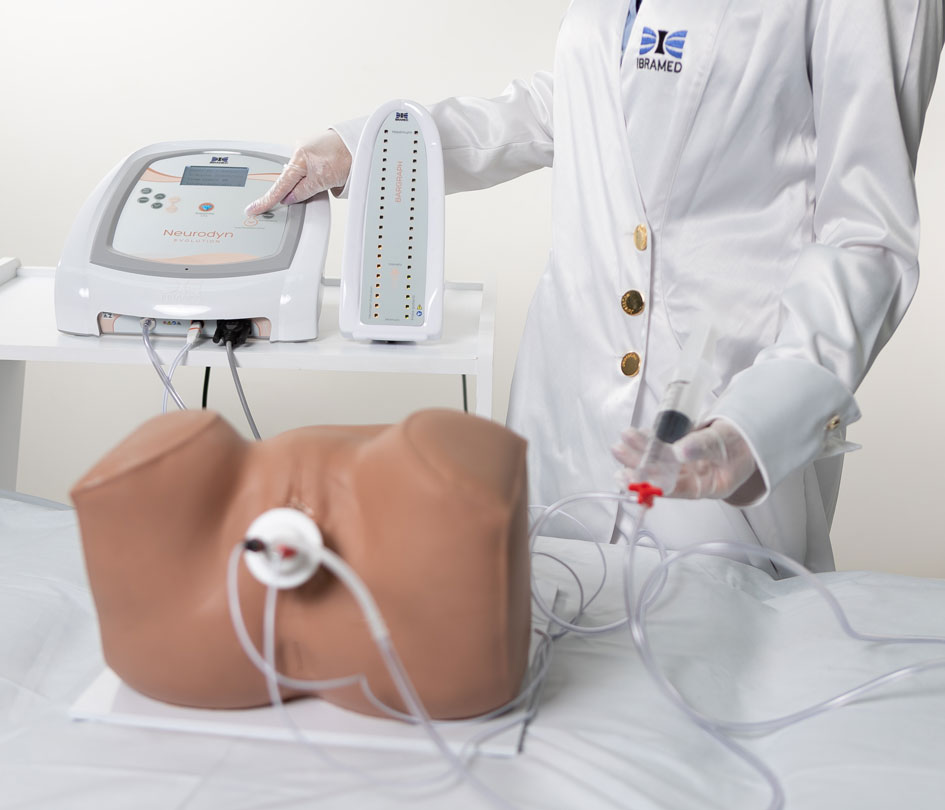
Bargraph
Tower
Includes a Bargraph Tower that shows perineal contraction force information by measuring air pressure levels inside the probe.

Neurodyn Evolution by IBRAMED is a device for aesthetic, physiotherapy, and rehabilitation treatments of urogynaecological and coloproctological dysfunctions via TENS, FES, Aussie, or Biofeedback therapies. It delivers transcutaneous, transanal, or transvaginal electrical stimulation via conductive silicone electrodes or a Teflon probe. It also features manometric-perineal biofeedback using a latex probe and a Bargraph Tower to visualize contraction force via internal air pressure levels.
FES is indicated for prevention or treatment of disuse atrophy, increased local circulation, muscle re-education, maintaining or increasing range of motion, and relaxation of spastic muscles. TENS is indicated for symptomatic relief and treatment of chronic pain, increased local circulation, symptomatic relief of acute post-traumatic pain, and acute postoperative pain.
Indications
- Strengthening of pelvic floor muscles
- Urinary incontinence
- Overactive bladder
- Hypertonia of pelvic floor muscles
- Dysfunction of pelvic floor muscles
- Fecal incontinence
Accessories
- 1× PP female IEC cable — 2 × 0.75 × 1500 mm
- 2× Conductive rubber electrodes 5 × 5 cm
- 1× Latex anal probe
- 1× Latex vaginal probe
- 1× Small vaginal Teflon probe
- 1× Vaginal Teflon probe
- 1× Anal Teflon probe
- 1× Vaginal biofeedback probe
- 1× Neurodyn Evolution Bargraph
- 1× Three-way valve for Evolution
- 1× White dual electrostimulation cable — red and black pins
- 1× Gel tube — 100 g capacity
Technical Characteristics
- ANVISA Registration: 10360319012
- Dimensions: Height 11.4 cm, Width 27 cm, Depth 29.4 cm
- Net Weight: 1.620 kg
- Voltage: Bivolt (110–240 V) | 50/60 Hz
Dúvidas frequentes sobre este equipamento IBRAMED
Quais técnicas podem ser utilizadas com esse equipamento?
Como devo proceder para realizar a limpeza adequada dos meus acessórios?
Permite aplicações em quais áreas?
Qual é o tempo de garantia oferecido?
Informações Técnicas
- Lipólise - Redução de gordura.
- Retração da derme.
- Estímulo de colágeno.
- Efeito lifting.
- Melhora da textura da pele.
- Região íntima.
Dimensões
- Largura 32cm
- Altura 21,5cm
- Profundidade 30cm
- Peso 8kg (incluindo case de transporte)
Voltagem
- Bivolt (110 - 220v)
Meu equipamento possui suporte na instalação?
Informações Técnicas
- Lipólise - Redução de gordura.
- Retração da derme.
- Estímulo de colágeno.
- Efeito lifting.
- Melhora da textura da pele.
- Região íntima.
Dimensões
- Largura 32cm
- Altura 21,5cm
- Profundidade 30cm
- Peso 8kg (incluindo case de transporte)
Voltagem
- Bivolt (110 - 220v)
Como posso ter treinamento de uso do equipamento?
Informações Técnicas
- Lipólise - Redução de gordura.
- Retração da derme.
- Estímulo de colágeno.
- Efeito lifting.
- Melhora da textura da pele.
- Região íntima.
Dimensões
- Largura 32cm
- Altura 21,5cm
- Profundidade 30cm
- Peso 8kg (incluindo case de transporte)
Voltagem
- Bivolt (110 - 220v)